Energy efficiency of lime brining
Practical Action
This can be presented mathematically by the efficiency equation:
Where
E
E
=
= Hc x Ls
Cf x Mf
Efficiency of the burning process
Hc = Theoretical heat of calcination per tonne for pure quicklime,
CaO plus MgO (MJ/tonne)
Ls = Available lime content (as CaO and MgO) of the quicklime
Cf = Calorific value of fuel (MJ/kg)
Mf = Mass of fuel used per tonne of quicklime (kg/tonne)
NB: Take care when using this formula that the units used are as specified or, if different
units are used, that mathematical consistency is maintained.
Explanation and derivation of terms
Hc
For all practical purposes, this can be taken as 3,200 MJ/tonne CaO for a pure calcite
limestone. For a pure dolomite limestone the figure is lower at 3,020 MJ. It is reasonably
straight forward to adjust the figure for 'dolomitic' limestones as follows in the example.
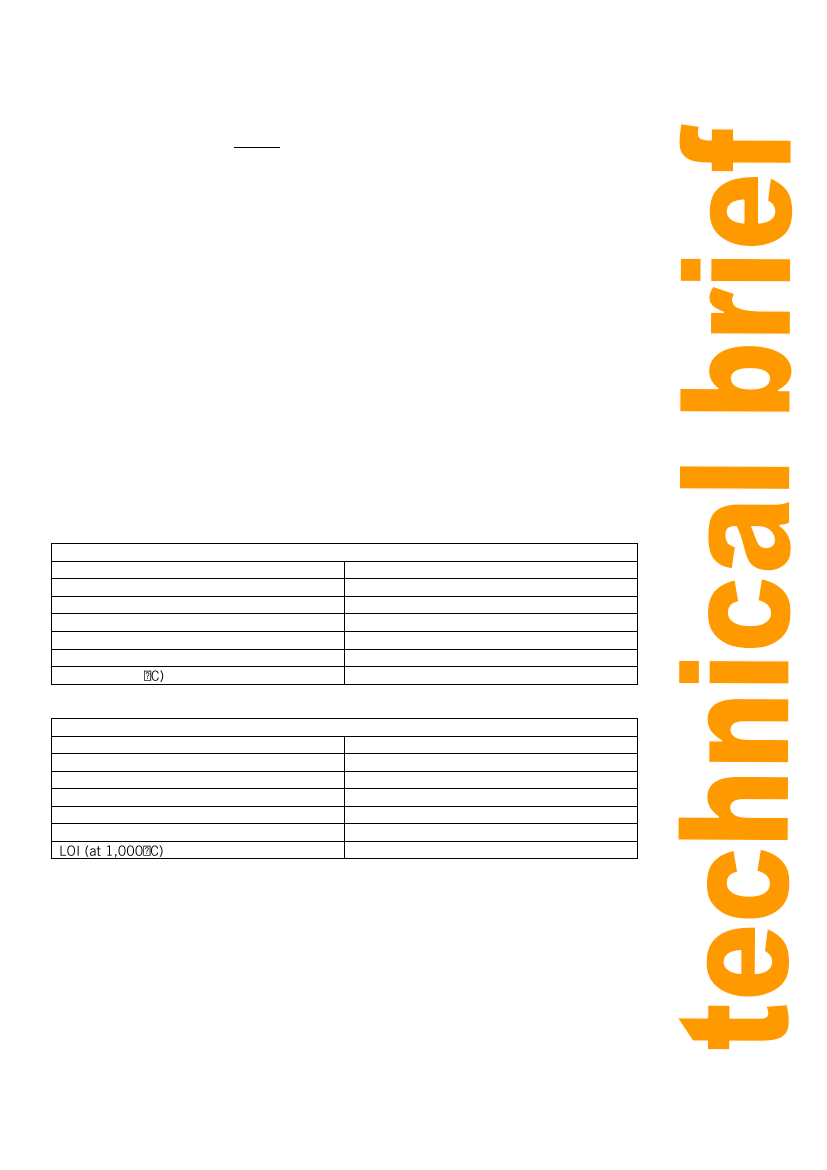
From a test on limestone the following chemical analysis was obtained:
Table 1: Chemical analysis obtained on limestone (%)
Silicon oxide, SiO2
2.03%
Aluminium Oxide, Al2O3
0.67%
Iron Oxide, Fe2O3
0.33%
Calcium Oxide, CaO
45.50%
Magnesium Oxide MgO
8.16%
Sulphuric Anhydride, SO3
0.39%
LOI (at 1,000
42.40%
A test on the quicklime produced the following result:
Table 2: Chemical analysis obtained on limestone (%)
Silicon oxide, SiO2
2.34%,
Aluminium Oxide, Al2O3
0.63,
Iron Oxide, Fe2O3
Calcium Oxide, CaO
0.35%,
68.40%,
Magnesium Oxide MgO
17.8%,
Sulphuric Anhydride, SO3
0.25%,
9.80%
Hc is obtained by calculating the energy required to calcine the calcitic fraction of the
limestone and that required to calcine the dolomitic fraction, and adding these two figures.
For the limestone in our example, the calcium oxide and magnesium oxide values (45.50%
and 8.16% respectively) indicate the stone has 60.95% calcite and 37.32% dolomite. These
values are obtained using the molecular weights of CaO, MgO, CaCO3 and MgCO3.(Table 3)
Thus,
Hc = (3200 x 0.6095) + (3020 x 0.3732) = 3,078 MJ
3